Introducing Harbor EDC
Harbor EDC combines compliance with a reimagined electronic data capture experience powered by AI.
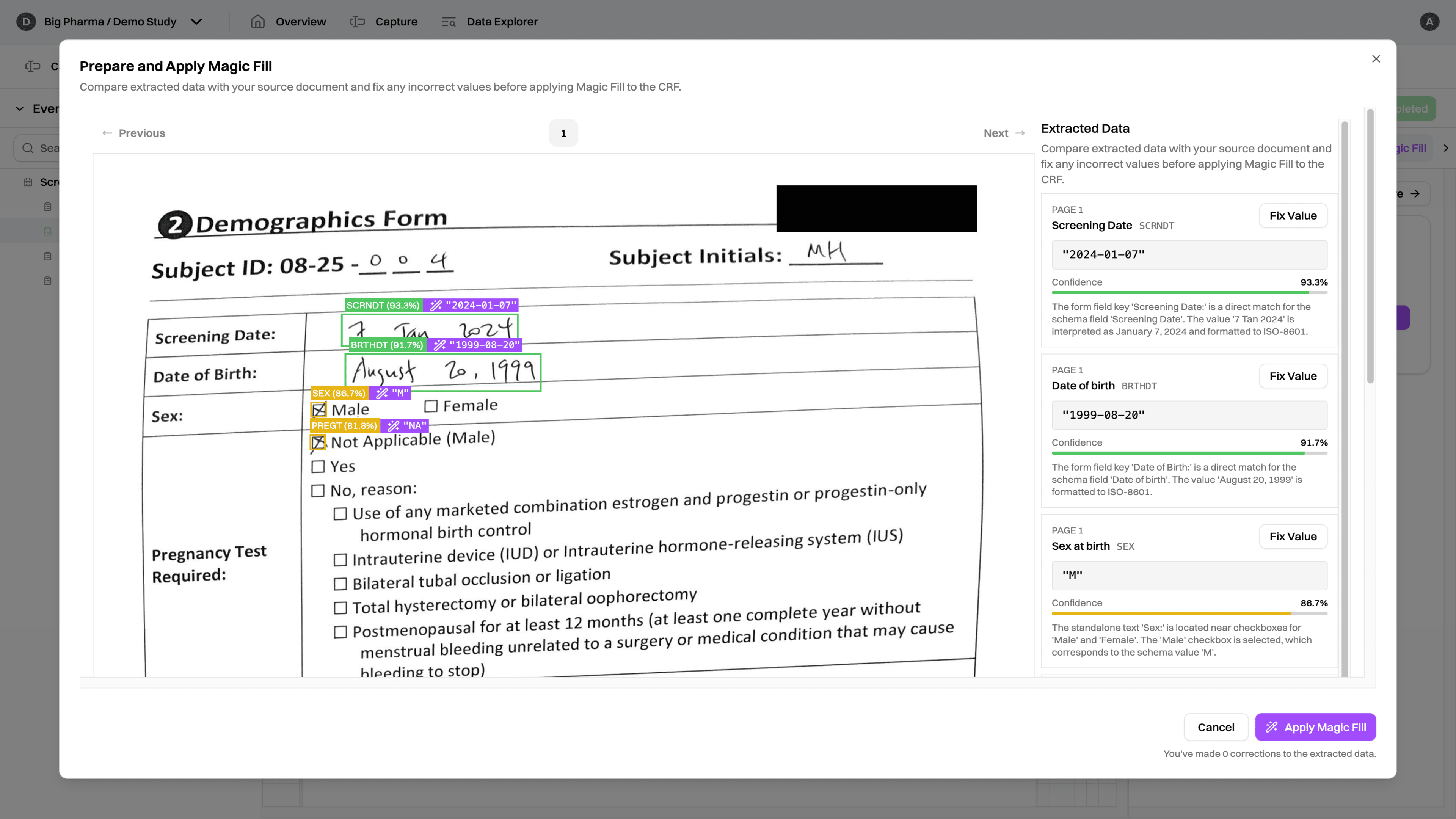
Demographics Form
Demographics
YSI 2300 STAT PLUS
In-Clinic Day
Blood Draw #1
0
Subjects Identified
Subject 102-003
Subject 205-017
Subject 118-024
Demographics Form
Demographics
Electronic Signature Record
Signed by: nleung Date: 2025-07-17 15:32:45 UTC Reason: Data entry completion and review
Electronic Signature
Your signature is the legally binding equivalent of your handwritten signature.
Submission-ready data. Faster than ever.
From protocol to CRF in minutes
LLMs turn clinical protocols into eCRFs within minutes, complete with edit checks, validations, and conditional visibility rules.
Demographics Form
Demographics
LLMs turn clinical protocols into eCRFs within minutes, complete with edit checks, validations, and conditional visibility rules.
Security
Your data, your rules.
Your Data
We create separate, highly-available, encrypted databases for every clinical trial.
Every trial has its own database, keys, and access controls. Data is protected by AES-256 encryption at rest and TLS 1.2 in transit. Everything is redundantly stored alongside Part 11-compliant audit logs in multiple data centers to ensure your data is always audit-ready and available.
Your Rules
We don't use your organization's data for anything other than running your studies.
Your organization's data is only used for the clinical trials you run. We have zero-data retention (ZDR) and business associate agreements (BAA) with all of our partners to ensure privacy, security, and compliance.
Features
Harbor is fast, compliant, and scalable.
Fast
Fast to learn, faster to use.
Harbor's intuitive, modern application design shortens onboarding for sites and sponsors, while our AI-powered features make data entry more accurate and data monitoring more efficient.
Compliant
Compliance built in
Live, comprehensive audit trails, sophisticated role-based permissions, and integrated tools for source data verification keep your study data clean and submission-ready.
Scalable
Pricing that scales with you
Scale from startup to enterprise with transparent, usage-based pricing models. Contact us to schedule a demo and get a custom quote.